Styrene CAS#100-42-5
Styrene CAS#100-42-5 Promotion Season Now in Store and Free Sample for Testing with Factory Price
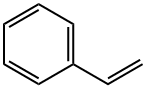
Chemical Name:Styrene
CAS No.: 100-42-5
Molecular Formula:C8H8
Molecular weight:104.15
Sample: Available
Mode of Transportation
1. By Air, fast but expensive.
2. By Sea, usual and economy.
3. By Train, suit for middle Asia countries.
4. By Express, suit for small package.
We only provide highest quality goods available, accompanied by after support!
Styrene CAS#100-42-5
Styrene is a positive natural chemical compound having the chemical components C8H8 and structural system CH2=CHC6H5, additionally recognized as styrol, vinylbenzene, phenylethene,phenylethylene,styrene,styreen and so on. Its chemical shape is made up of a benzene ring bonded onto a vinyl team . At room temperature and pressure, styrene is a clear, colorless liquid. Styrene is an necessary monomer of synthetic rubber, adhesives and plastics, such as styrene sheet.

Styrene Chemical Properties |
Melting point | -31 °C (lit.) |
Boiling point | 145-146 °C (lit.) |
density | 0.906 g/mL at 25 °C |
vapor density | 3.6 (vs air) |
vapor pressure | 12.4 mm Hg ( 37.7 °C) |
refractive index | n |
Fp | 88 °F |
storage temp. | Store at <= 20°C. |
solubility | 0.24g/l |
form | Liquid |
pka | >14 (Schwarzenbach et al., 1993) |
Specific Gravity | 0.909 |
color | Colorless |
Odor | at 0.10 % in triacetin. sweet balsam floral plastic |
Odor Type | balsamic |
Odor Threshold | 0.035ppm |
explosive limit | 1.1-8.9%(V) |
Water Solubility | 0.3 g/L (20 ºC) |
FreezingPoint | -30.6℃ |
Sensitive | Air Sensitive |
Merck | 14,8860 |
BRN | 1071236 |
Henry's Law Constant | (x 10-3 atm?m3/mol): 3.91 at 25 °C (static headspace-GC, Welke et al., 1998) |
Exposure limits | TLV-TWA 50 ppm (~212 mg/m3) (ACGIH and NIOSH), 100 ppm (~425 mg/m3) (OSHA and MSHA); ceiling 200 ppm, peak 600 ppm/5 min/3 h (OSHA); STEL 100 ppm (~425 mg/m3) (ACGIH). |
Dielectric constant | 2.4(25℃) |
Stability: | Stable, but may polymerize upon exposure to light. Normally shipped with a dissolved inhibitor. Substances to be avoided include strong acids, aluminium chloride, strong oxidizing agents, copper, copper alloys, metallic salts, polymerization catalysts and accelerators. Flammable - vapour may travel considerable distance to ignition source |
InChIKey | PPBRXRYQALVLMV-UHFFFAOYSA-N |
LogP | 2.96 at 25℃ |
CAS DataBase Reference | 100-42-5(CAS DataBase Reference) |
IARC | 2A (Vol. 60, 82, 121) 2019 |
NIST Chemistry Reference | Styrene(100-42-5) |
EPA Substance Registry System | Styrene (100-42-5) |
Safety Information |
Hazard Codes | Xn,T,F |
Risk Statements | 10-20-36/38-40-36/37/38-39/23/24/25-23/24/25-11-48/20-63 |
Safety Statements | 23-36-26-16-45-36/37-7-46 |
RIDADR | UN 2055 3/PG 3 |
OEB | A |
OEL | TWA: 50 ppm (215 mg/m3), STEL: 100 ppm (425 mg/m3) |
WGK Germany | 2 |
RTECS | WL3675000 |
Autoignition Temperature | 914 °F |
TSCA | Yes |
HS Code | 2902 50 00 |
HazardClass | 3 |
PackingGroup | III |
Hazardous Substances Data | 100-42-5(Hazardous Substances Data) |
Toxicity | LD50 in mice (mg/kg): 660 ± 44.3 i.p.; 90 ± 5.2 i.v. |
IDLA | 700 ppm |
Product Usage
Styrene is an necessary monomer of artificial rubber, adhesives and plastics. [3,4,5] It is used for the synthesis of styrene butadiene rubber and polystyrene resin, polyester glass fiber bolstered plastics and coatings. It is used for making ready polystyrene, ion change resin, and foam polystyrene. It is additionally used for copolymerization with different monomers to produce a number of engineering plastics, such as copolymerization of acrylonitrile and butadiene to produce ABS resin, broadly used in a number family home equipment and industries. Copolymerization with acrylonitrile, bought SAN is a resin with shock resistance and shiny color. The SBS produced by means of copolymerization with butadiene is a thermoplastic rubber, which is broadly used as a polyvinyl chloride and acrylic modifier. SBS and SIS thermoplastic elastomers are made with butadiene and isoprene copolymerization, and as a crosslinking monomer, styrene is used in the change of PVC, polypropylene, and unsaturated polyester.
Syrene is used as a challenging monomer for the manufacturing of styrene acrylic emulsion and solvent strain touchy adhesive. Emulsion adhesive and paint can be organized by using copolymerization with vinyl acetate and acrylic ester. Styrene is one of the most regularly used vinyl monomers in the scientific field, used in a number modified and composite materials.[6]
In addition, a small quantity of styrene is additionally used as fragrance and different intermediates. By chloromethylation of styrene, cinnamyl chloride is used as an intermediate for the non anesthetic analgesic sturdy ache determination, and styrene is additionally used as an antitussive, expectorant and anticholinergic unique medication in belly Changning. It can be used to synthesize anthraquinones dye intermediates , pesticide emulsifiers, and styrene phosphonic acids ore dressing agent and copper plating brighteners.[7]
Factory and Equipment Show


Fast transport time
Inventory 2-3 working days New manufacturing 7-10 working days

Related Products
![2,2'-[(1-Methylethylidene)bis[(dibromo-4,1-phenylene)oxymethylene]]bis[oxirane]-4,4'-(1-methylethylidene)bis[2,6-dibromophenol] copolymer CAS#68928-70-1](https://sdluxicdn.huazhi.cloud/cdn/ff/XFgEQtl6DCXivDewGGVrfwI60D3eh_vhrnIzsWOMHt0/1717742405/public/styles/chanp/public/2024-06/%E6%97%A0%E8%89%B2%E6%B6%B2%E4%BD%93%20%283%29%20-%20%E5%89%AF%E6%9C%AC_5.jpg?itok=dhUiUgCC)