Adenosine5'-(tetrahydrogen triphosphate), disodiuM salt, trihydrate (9CI) CAS#51963-61-2
Adenosine5'-(tetrahydrogen triphosphate), disodiuM salt, trihydrate (9CI) CAS#51963-61-2 Promotion Season Now in Store and Free Sample for Testing with Factory Price
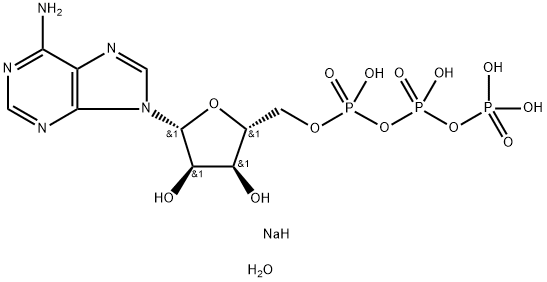
Chemical Name:Adenosine5'-(tetrahydrogen triphosphate), disodiuM salt, trihydrate (9CI)
CAS No.:51963-61-2
Molecular Formula:C10H19N5NaO14P3
Molecular weight:549.19
Sample: Available
Mode of Transportation
1. By Air, fast but expensive.
2. By Sea, usual and economy.
3. By Train, suit for middle Asia countries.
4. By Express, suit for small package.
We only provide highest quality goods available, accompanied by after support!
Products Description of Adenosine5'-(tetrahydrogen triphosphate), disodiuM salt, trihydrate (9CI) CAS#51963-61-2
This product is white crystal or off-white powder, odorless, slightly sour, and hygroscopic. It is easily soluble in water and very slightly soluble in ethanol, ether, chloroform and benzene. It is relatively stable in alkaline solutions and easily decomposed in acidic and neutral solutions. It is also easily decomposed by heat.
Adenosine5'-(tetrahydrogen triphosphate), disodiuM salt, trihydrate (9CI) Chemical Properties
Melting point | 176°C |
storage temp. | 2-8°C |
solubility | H2O: 50 mg/mL |
form | lyophilized powder |
Water Solubility | Water: 250 mg/mL (413.09 mM) |
InChIKey | MWEQTWJABOLLOS-AZGWGOJFSA-L |
CAS DataBase Reference | 51963-61-2(CAS DataBase Reference) |
Safety Information
Hazard Codes | Xi |
Risk Statements | 36/37/38 |
Safety Statements | 22-24/25-36-26 |
WGK Germany | 3 |
RTECS | AU7417000 |
TSCA | Yes |
Product Application of Adenosine5'-(tetrahydrogen triphosphate), disodiuM salt, trihydrate (9CI) CAS#51963-61-2
When used for myocardial infarction and cerebral hemorrhage, it should be started 2-3 weeks after the onset of the disease. There are fewer side effects; intravenous injection should be slow.
Factory and Equipment Show


Fast delivery time
Inventory 2-3 working days New production 7-10 working days