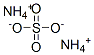Ammonium sulfate CAS#7783-20-2
Ammonium sulfate CAS#7783-20-2 Promotion Season Now in Store and Free Sample for Testing with Factory Price
Chemical Name:Ammonium sulfate
CAS No.:7783-20-2
Molecular Formula:H8N2O4S
Molecular weight:132.14
Sample: Available
Mode of Transportation
1. By Air, fast but expensive.
2. By Sea, usual and economy.
3. By Train, suit for middle Asia countries.
4. By Express, suit for small package.
We only provide highest quality goods available, accompanied by after support!
Products Description of Ammonium sulfate CAS#7783-20-2
Ammonium sulfate, also known as ammonium sulfate, is the earliest nitrogen fertilizer produced and used at home and abroad. It is usually regarded as a standard nitrogen fertilizer with a nitrogen content of 20% to 30%. Ammonium sulfate is a salt of strong acid and weak base, and its aqueous solution is acidic. Ammonium sulfate is a nitrogen fertilizer and an acidic fertilizer among inorganic fertilizers. Long-term single use will cause soil acidification and hardening, which needs to be improved. Ammonium sulfate cannot be used to produce organic fertilizers. Moreover, acidic fertilizers cannot be used together with alkaline fertilizers, and double hydrolysis can easily cause the fertilizer effect to dissipate. In the 1960s, ammonium sulfate was the main variety of nitrogen fertilizer and one of the main sources of sulfur for crop nutrients. It was first produced by neutralizing ammonia and sulfuric acid, but later the proportion of by-product ammonium sulfate became larger and larger. Now, domestically, it is basically all by-products from other industries, such as coking industry, caprolactam, sulfuric acid tail gas desulfurization, power plant desulfurization, acrylonitrile, methyl methacrylate, zinc oxide and other by-products. By-product ammonium sulfate follows the principle of "waste treatment with waste", realizes the comprehensive utilization of waste, and achieves the purpose of energy conservation and emission reduction. Especially with the technological breakthrough of ammonia-based desulfurization projects, the output of ammonium sulfate as a by-product of desulfurization in power plants will increase significantly in the future.
Ammonium sulfate Chemical Properties
Melting point | >280 °C (dec.) (lit.) |
density | 1.77 g/mL at 25 °C (lit.) |
vapor pressure | <1 Pa (25 °C) |
refractive index | n20/D 1.396 |
Fp | 26 °C |
storage temp. | room temp |
solubility | H2O: 1 M at 20 °C, clear, colorless |
form | Solid |
Specific Gravity | 1.769 |
color | Yellow to orange |
Odor | Slight odor of ammonia |
PH Range | 5 - 6 |
PH | 5.0-6.0 (25℃, 1M in H2O) |
Water Solubility | 77 g/100 mL (25 ºC) |
λmax | λ: 260 nm Amax: ≤0.037 |
Merck | 14,555 |
Stability: | Stable. Contact with strong oxidizers may cause fire or explosion. Incompatible with strong bases. |
InChIKey | BFNBIHQBYMNNAN-UHFFFAOYSA-N |
LogP | -5.1 at 25℃ |
CAS DataBase Reference | 7783-20-2(CAS DataBase Reference) |
EPA Substance Registry System | Ammonium sulfate (7783-20-2) |
Safety Information
Hazard Codes | Xi,Xn |
Risk Statements | 10-36/37/38-22 |
Safety Statements | 37/39-26-36-24/25 |
WGK Germany | 1 |
RTECS | BS4500000 |
TSCA | Yes |
HS Code | 31022100 |
Hazardous Substances Data | 7783-20-2(Hazardous Substances Data) |
Toxicity | LD50 orally in Rabbit: 2840 mg/kg |
Product Application of Ammonium sulfate CAS#7783-20-2
Biological preparations, protein precipitation, chromatography reagents, urine tests, preparation of culture medium in haploid breeding. Pharmaceuticals use it as a salting-out agent, a catalyst for food coloring, and for the cultivation of yeast in fresh yeast production. It is also used as an auxiliary dye for acid dyes and a deliming agent for leather. Hydrogen peroxide is produced to make ammonium persulfate, as well as for electroplating, etc.
Factory and Equipment Show


Fast transport time
Inventory 2-3 working days New manufacturing 7-10 working days