Maltose CAS#69-79-4
Maltose CAS#69-79-4 Promotion Season Now in Store and Free Sample for Testing with Factory Price
Chemical Name:Maltose
CAS No.:69-79-4
Molecular Formula:C12H22O11
Molecular weight:342.3
Sample: Available
Mode of Transportation
1. By Air, fast but expensive.
2. By Sea, usual and economy.
3. By Train, suit for middle Asia countries.
4. By Express, suit for small package.
We only provide highest quality goods available, accompanied by after support!
Products Description of Maltose CAS#69-79-4
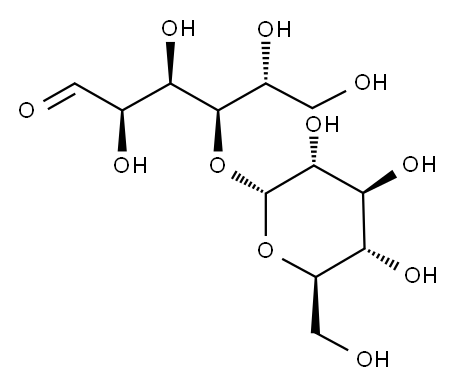
Maltose is the abbreviation of maltobiose, also known as D-maltose and maltose. A disaccharide formed by connecting, condensing, and dehydrating two molecules of α-D-glucose through α-1,4 glycosidic bonds. There is also a free hemiacetal hydroxyl group in the molecule, which is a reducing sugar. Like glucose, it can make Doron's reagent and Fehling's reagent react positively. These α-1,4-glucoside bonds are connected to form a long chain, which is called amylose, with an average molecular weight of 20,000 to 200,000.

Maltose Chemical Properties
Melting point | 110 °C |
Boiling point | 397.76°C (rough estimate) |
density | 1.5400 |
refractive index | n20/D 1.361 |
storage temp. | 2-8°C |
solubility | Very soluble in water; very slightly soluble in cold ethanol (95%); practically insoluble in ether. |
pka | 12.39±0.20(Predicted) |
PH | 4.0-6.5 (25℃) |
Water Solubility | 310.3g/L(20 ºC) |
λmax | λ: 260 nm Amax: 0.08 |
Merck | 13,5736 |
BRN | 93798 |
Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. |
LogP | -3.391 (est) |
CAS DataBase Reference | 69-79-4(CAS DataBase Reference) |
NIST Chemistry Reference | D-Glucose, 4-o-«alpha»-D-glucopyranosyl-(69-79-4) |
EPA Substance Registry System | D-Glucose, 4-O-.alpha.-D-glucopyranosyl- (69-79-4) |
Safety Information
WGK Germany | - |
Product Application of Maltose CAS#69-79-4
Scientific research and experiments. There is an aldehyde group in the molecular structure of germose, which has reducing properties and is a reducing sugar. Therefore, it can react with silver ammonia solution to produce a silver mirror, or it can react with newly prepared alkaline copper hydroxide to form a brick-red precipitate. It can be hydrolyzed under certain conditions to produce two molecules of glucose. Used as biological culture medium, polysulfide determination agent, analytical chemistry colorimetric determination standard brown
Factory and Equipment Show


Fast delivery time
Inventory 2-3 working days New production 7-10 working days