Lithium Aluminum Hydride CAS#16853-85-3
Lithium Aluminum Hydride CAS#16853-85-3 Promotion Season Now in Store and Free Sample for Testing with Factory Price
Chemical Name:Lithium Aluminum Hydride
CAS No.:16853-85-3
Molecular Formula:LiAlH4
Molecular weight:37.954298
Sample: Available
Mode of Transportation
1. By Air, fast but expensive.
2. By Sea, usual and economy.
3. By Train, suit for middle Asia countries.
4. By Express, suit for small package.
We only provide highest quality goods available, accompanied by after support!
Products Description of Lithium Aluminum Hydride CAS#16853-85-3
Lithium aluminum hydride is a commonly used reducing agent in organic chemistry. It can reduce a variety of functional group compounds.
It can also act on double-bond and triple-bond compounds to achieve hydroalumination. In addition, lithium aluminum hydride can also participate in the reaction as a base.
For example, react LiAlH4 with 2 mol of ethanol to generate lithium diethoxyaluminum hydride [LiAlH2(OC2H5)2];
react with 3 mol of tert-butanol to generate lithium tri-tert-butoxyaluminum LiAlH[OC(CH3)3]3. Lithium aluminum hydride has a strong hydrogen transfer ability and can reduce aldehydes, ketones, esters, lactones, carboxylic acids, anhydrides and epoxides to alcohols, or convert amides, imine ions, nitriles and aliphatic nitro compounds to corresponding amines. In addition, the super strong reducing ability of lithium aluminum hydride allows it to act on other functional groups, such as reducing halogenated alkanes to alkanes.
In this type of reaction, the activity of the halides is from large to small, followed by iodide, bromide and chloride.
Lithium Aluminum Hydride Chemical Properties
Melting point | 125 °C (dec.)(lit.) |
Boiling point | 0°C |
density | 0.97 g/mL at 20 °C |
Fp | 99 °F |
storage temp. | Store below +30°C. |
solubility | reacts with H2O, ethanol; seth, tetrahydrofuran |
form | tablets (~0.5 g each) |
color | White to light gray |
Specific Gravity | 0.917 |
Odor | Odorless solid |
Water Solubility | Reacts |
Sensitive | Air & Moisture Sensitive |
Hydrolytic Sensitivity | 8: reacts rapidly with moisture, water, protic solvents |
Merck | 14,350 |
Exposure limits | TLV-TWA 2 mg(Al)/m3 (ACGIH). |
Stability: | Stable. Reacts violently with water, liberating hydrogen. Incompatible with strong oxidizing agents, alcohols, acids. |
CAS DataBase Reference | 16853-85-3(CAS DataBase Reference) |
NIST Chemistry Reference | Lithium tetrahydroaluminate(16853-85-3) |
EPA Substance Registry System | Lithium aluminum hydride (16853-85-3) |
Safety Information
Hazard Codes | F,C,Xi,Xn,F+,T |
Risk Statements | 15-34-14/15-11-36/37-19-40-10-67-66-22-12-35-37-65-48/20-63-36/38-61-60 |
Safety Statements | 43-7/8-6A-45-43B-36/37/39-33-26-16-24/25-27-29-62-53 |
RIDADR | UN 3399 4.3/PG 1 |
WGK Germany | 2 |
RTECS | BD0100000 |
F | 10-21 |
TSCA | Yes |
HS Code | 2850 00 20 |
HazardClass | 4.3 |
PackingGroup | I |
Hazardous Substances Data | 16853-85-3(Hazardous Substances Data) |
Toxicity | TLV-TWA (ACGIH) 2 mg (Al)/m3 |
Product Application of Lithium Aluminum Hydride CAS#16853-85-3
It is used as a polymerization catalyst, reducing agent, jet fuel, and also for the synthesis of drugs. Lithium aluminum hydride is an extremely strong reducing agent. It can reduce aldehydes, ketones, acids, anhydrides, esters, lactones, quinones, acyl chlorides, etc. to alcohols, nitriles to primary amines, and halogenated hydrocarbons to hydrocarbons. However, it usually cannot hydrogenate carbon-carbon double bonds. It is widely used as a reducing agent in medicine, fragrances, pesticides, dyes, and other fine organic syntheses.
Factory and Equipment Show


Fast delivery time
Inventory 2-3 working days New production 7-10 working days




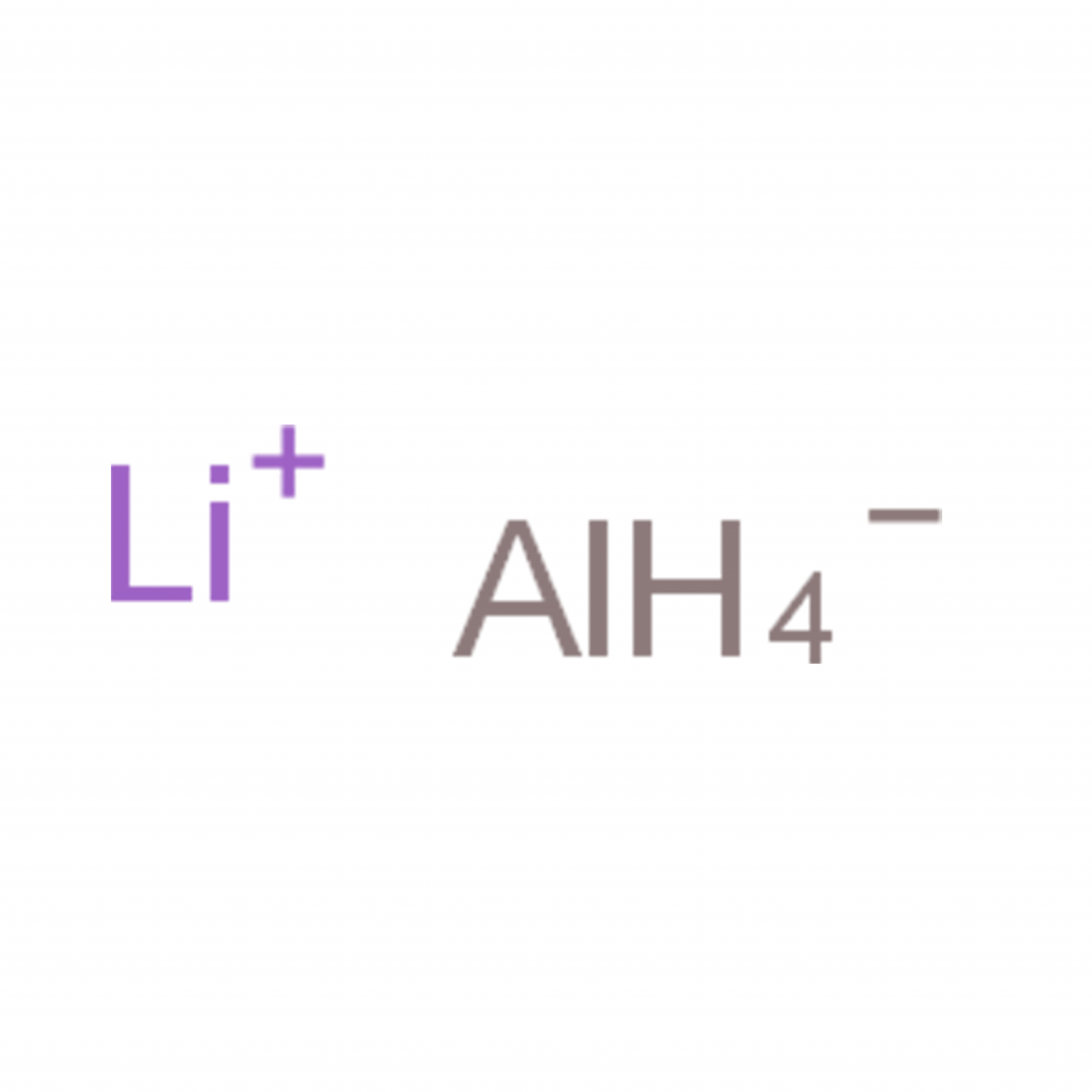


![N-[3-(Trimethoxysilyl)propyl]ethylenediamine CAS#1760-24-3](https://sdluxicdn.huazhi.cloud/cdn/ff/-eD49wJJiTJAvEfl-FDfyLeQmMCCDZH69M_88oGd2WY/1716289027/public/styles/chanp/public/2024-05/%E6%97%A0%E8%89%B2%E6%B6%B2%E4%BD%93%20%282%29_20.jpg?itok=JvS0Sjd4)

