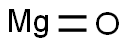Magnesium oxide CAS#1309-48-4
Magnesium oxide CAS#1309-48-4 Promotion Season Now in Store and Free Sample for Testing with Factory Price
Chemical Name:Magnesium oxide
CAS No.1309-48-4
Molecular Formula:MgO
Molecular weight:40.3
Sample: Available
Mode of Transportation
1. By Air, fast but expensive.
2. By Sea, usual and economy.
3. By Train, suit for middle Asia countries.
4. By Express, suit for small package.
We only provide highest quality goods available, accompanied by after support!
Products Description of Magnesium oxide CAS#1309-48-4
Magnesium oxide is commonly known as magnesia, also known as magnesium oxide. It is a typical alkaline earth metal oxide with the chemical formula MgO. It is a white powder with a melting point of 2852℃, a boiling point of 3600℃ and a relative density of 3.58 (25℃). It is soluble in acid and ammonium salt solutions. It reacts slowly with water to form magnesium hydroxide. It can be dissolved in a carbon dioxide aqueous solution to form magnesium bicarbonate. It can gradually absorb water and carbon dioxide in the air, and emit irritating smoke when heated. Magnesite (MgCO3), dolomite (MgCO3·CaCO3) and seawater are the main raw materials for the production of magnesium oxide. Magnesium oxide is obtained by thermal decomposition of magnesia or dolomite. Magnesium hydroxide precipitate is obtained by treating seawater with slaked lime, and magnesium hydroxide is burned to obtain magnesium oxide. Magnesium chloride brine blocks obtained from the comprehensive utilization of seawater or brine after bromine extraction can also be used as raw materials, sodium hydroxide or sodium carbonate are added to generate magnesium hydroxide or basic magnesium carbonate precipitate, and then burned to obtain magnesium oxide. At present, China mainly uses magnesia, dolomite, brine or brine blocks as raw materials. Magnesium oxide has the largest output among magnesium compounds, accounting for about 3/4 of all magnesium industrial products. Magnesium oxide produced below 900℃ is light magnesium oxide, with low density, large specific surface area and strong adsorption. It can be used as a catalyst, rubber filler and accelerator to improve rubber performance. It is mixed with magnesium chloride solution to make magnesium cement. It is used as a flame retardant for building materials. It is used as an antacid and laxative in medicine for hyperacidity and gastric and duodenal ulcers, and is often used in combination with calcium carbonate that is prone to constipation. It is used as an animal feed additive and plant fertilizer. The light magnesium oxide produced at 950-1050℃ has a large density, a certain range of particle distribution, and is easier to combine with water. It can be used as an isolation agent for silicon steel plates to prevent sintering and adhesion during high-temperature treatment by reacting with silicon dioxide on the surface of silicon steel at high temperature to form a magnesium silicate film. Heavy magnesium oxide produced at 1500-1800℃ has high density, small specific surface area, is not easily decomposed by heat, has low chemical activity, is not easy to react with acid, and has low hydration rate. It is used as high-temperature refractory material, binder for making refractory crucibles and furnace linings, etc.
Magnesium oxide Chemical Properties
Melting point | 2852 °C (lit.) |
Boiling point | 3600 °C |
density | 3.58 |
refractive index | 1.736 |
Fp | 3600°C |
storage temp. | no restrictions. |
solubility | 5 M HCl: 0.1 M at 20 °C, clear, colorless |
form | nanopowder |
color | White |
Specific Gravity | 3.58 |
PH | 10.3 (H2O, 20℃)(saturated solution) |
Odor | wh. powd. or cryst., odorless |
Resistivity | 1.3 ∞ 10*15 (ρ/μΩ.cm) |
Water Solubility | 6.2 mg/L (20 ºC), reacts |
Sensitive | Air Sensitive |
λmax | λ: 260 nm Amax: ≤0.040 |
Crystal Structure | Cubic |
Merck | 14,5677 |
Exposure limits | ACGIH: TWA 10 mg/m3 |
Dielectric constant | 9.7(0.0℃) |
Stability: | Stable. Incompatible with bromine trifluoride, bromine trichloride, phosphorus pentachloride. |
InChIKey | CPLXHLVBOLITMK-UHFFFAOYSA-N |
CAS DataBase Reference | 1309-48-4(CAS DataBase Reference) |
NIST Chemistry Reference | Magnesium monoxide(1309-48-4) |
EPA Substance Registry System | Magnesium oxide (1309-48-4) |
Safety Information
HS Code | 25199099 |
Hazardous Substances Data | 1309-48-4(Hazardous Substances Data) |
Toxicity | TCLo inhalation in human: 400mg/m3 |
IDLA | 750 mg/m3 |
Product Application of Magnesium oxide CAS#1309-48-4
1. Light magnesium oxide is used in ceramics, enamel, refractory crucibles, refractory bricks, etc. It is also used as a polishing agent, adhesive, paint and papermaking filler, promoter and activator of chloroprene rubber. It is used as an antacid and laxative in medicine, used for gastric acid hyperacidity and duodenal ulcer, and also used in glass dyes, phenolic acid, plastics and other industries.
2. Dead burned magnesium oxide is magnesia, which is granular and brick-shaped. It is widely used as a refractory material for steelmaking furnaces, cement kilns, glass combustion furnaces, etc.
3. Alkaline granular refractory is mainly used in metal refining industry. Block refractory is used as furnace, or granular refractory is used as maintenance material. It is attached to the furnace wall by spraying or coating to enhance the refractory capacity of the furnace.
4. Magnesium oxide generates positive charge in water, and most suspended matter is negatively charged, which plays an absorption role and can improve the filtering effect.
Factory and Equipment Show


Fast delivery time
Inventory 2-3 working days New production 7-10 working days