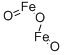Ferric oxide CAS# 1309-37-1
Ferric oxide CAS# 1309-37-1Promotion Season Now in Store and Free Sample for Testing with Factory Price
Chemical Name:Ferric oxide
CAS No.:1309-37-1
Molecular Formula:Fe2O3
Molecular weight:159.69
Sample: Available
Mode of Transportation
1. By Air, fast but expensive.
2. By Sea, usual and economy.
3. By Train, suit for middle Asia countries.
4. By Express, suit for small package.
We only provide highest quality goods available, accompanied by after support!
Products Description of Direct Ferric oxide CAS# 1309-37-1
Iron oxides are produced synthetically and consist essentially of anhydrous and/or hydrated iron oxides. The range of hues includesyellows, reds, browns and blacks. Food quality iron oxides are primarily distinguished from technical grades by the comparatively low levels of contamination by other metals. This is achieved by the selection and control of the source of iron and/or by the extent of chemical purification during the manufacturing process. Iron oxides have been used to color confectionery, fillings and decorations for pastry products, cheese products, fish paste, pet foods, cosmetics and pharmaceutical products.

Melting point | 1538°C |
density | 5.24 |
Fp | >230 °F |
storage temp. | 2-8°C |
solubility | It is soluble In Warm Hydrochloric Acid, Slightly Soluble in Sulfuric Acid. |
form | pieces |
color | black |
Specific Gravity | 5.1~5.2 |
PH | 3.7±0.3 |
Water Solubility | INSOLUBLE |
Crystal Structure | Corundum type |
crystal system | Three sides |
Merck | 14,4028 |
Space group | R3c |
Lattice constant | a/nmb/nmc/nmα/oβ/oγ/oV/nm30.502590.502591.373590901200.3005 |
Exposure limits | ACGIH: TWA 5 mg/m3 |
Stability: | Stable. |
CAS DataBase Reference | 1309-37-1(CAS DataBase Reference) |
IARC | 3 (Vol. 1, Sup 7) 1987 |
NIST Chemistry Reference | Iron(iii) oxide(1309-37-1) |
EPA Substance Registry System | Ferric oxide (1309-37-1) |
Product Application of Direct Ferric oxide CAS# 1309-37-1
Red iron oxide (Fe2O3) is an inorganic pigment of either natural or synthetic origin. It is a low chroma red with excellent durability and low cost. Synthetic pigment is made by heating iron sulfate with quicklime in a furnace. The second preparatory technique involves calcining iron sulfate in the presence of air at high temperatures. Natural and oxides of iron are mined either as the mineral hematite (Fe2O3) or as hematite in its hydrated form.
Factory and Equipment Show


Fast transport time
Inventory 2-3 working days New manufacturing 7-10 working days