Methotrexate CAS#59-05-2
Methotrexate CAS#59-05-2 Promotion Season Now in Store and Free Sample for Testing with Factory Price
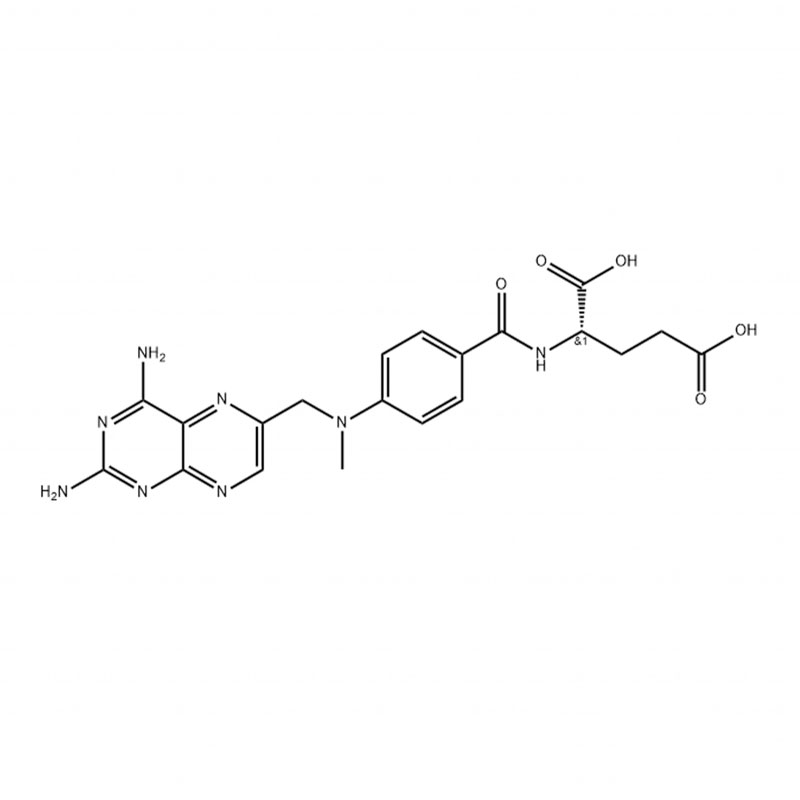
Chemical Name:Methotrexate
CAS No.:59-05-2
Molecular Formula: C20H22N8O5
Molecular weight: 454.45
Sample: Available
Mode of Transportation
1. By Air, fast but expensive.
2. By Sea, usual and economy.
3. By Train, suit for middle Asia countries.
4. By Express, suit for small package.
We only provide highest quality goods available, accompanied by after support!
Products Description of Methotrexate CAS#59-05-2
This product is a good anti-tumor drug and is used for acute lymphoblastic leukemia, lymphoma, malignant hydatidiform mole, choriocarcinoma, squamous cell carcinoma, lung cancer, cervical cancer, breast cancer and osteosarcoma Chemicalbook. 90% of the original drug of methotrexate is excreted in the urine 12 hours after oral administration, and the main metabolite is 7-hydroxymethotrexate. The LD50 of intravenous injection in rats is 14mg/kg.
Product Parameters of Methotrexate CAS#59-05-2
1. Names and Identifiers
Name | Methotrexate
|
Synonyms
| (+)-Amethopterin 4-amino-4-deoxy-10-methylpteroyl-L-Glutamic acid 4-Amino-N10-methylpteroylglutamic Acid EINECS 200-413-8 Hdmtx L-Amethopterin L-glutamic acid, N-(4-(((2,4-diamin0-6- pteridinyl)methyl)methylamino)benzoyl)- L-Glutamic acid, N-[4-[[(2,4-diamino-6- pteridinyl)methyl]methylamino]benzoyl]-(9Cl) METHOTREXATE(N-(4-(((2,4-DIAMINO-6- PTERIDINYL)METHYLAMINO)BENZOYL)-L-GLUTAMICACID) METHOTREXATEFORINJECTION MFCD00064370 N-(4-{[(2,4-Diamino-6-pteridinyl)methyl](methyl)amino}benzoyl)-L- glutamic acid |
2. Properties
Density
| 1.536
|
Melting point
| 195℃
|
Boiling point | 561.26°C (rough estimate)
|
Refractive index | 1.6910 (estimate)
|
Flash Point
| 11℃
|
Precise Quality
| 454.17100
|
PSA
| 210.54000
|
logP
| 1.82170
|
Solubility
| H2O: insoluble
|
Appearance
| yellow crystalline powder
|
Storage
| Store at -20°C. Hygroscopic. Light Sensitive.
|
Color/Form
| Pale-yellow to Yellow-brown Solid
|
Decomposition
| When heated to decomposition it emits toxic fumes including /nitrogen oxides/.
|
pKa | pKa 3.04/4.99(H2O,t =25,I=0.0025) (Uncertain)
|
Water Solubility | Insoluble. |
Spectral Properties
| Specific optical rotation: 20.4 + or - 0.6 deg at 21 deg C/589 D (concentration by 0.1 N sodium hydroxide) maximum absorption: 243nm, A1= 388;307 nm, A1 = 475 (in 0.1 n hydrogen chloride). 258 nm, A1= 544; 303 nm, A1= 546; 372 nm, A1= 177 (in 0.1 N sodium hydroxide)
|
Stability | Stable, but light sensitive and hygroscopic. Incompatible with strong acids, strong oxidizing agents. Store at -15C or below.
|
StorageTemp | −20°C |
3. Use and Manufacturing
3.1 Potential Exposure
Methotrexate is an alkaloid anticancerdrug available in tablet or injectable liquid form. A chemotherapy drug that interferes with DNA and RNA synthesis.It is also an insect chemosterilant.
3.2 Purification Methods
Most common impurities are 10-methylpteroylglutamic acid, aminopterin and pteroylglutamic acid. Purify it by chromatography on Dowex-1 acetate, followed by filtration through a mixture of cellulose and charcoal. It has been recrystallised from aqueous HCl or by dissolution in the minimum volume of N NaOH and acidified until precipitation is complete, filter or better collect by centrifugation, wash with H2O (also by centrifugation) and dry at 100o/3mm. It has UV: max at 244 and 307nm ( 17300 and 19700) in H2O at pH 1; 257, 302 and 370nm ( 23000, 22000 and 7100) in 2O at pH 13. [Momle Biochemical Preparations 8 20 1961, Seeger et al. J Am Chem Soc 71 1753 1949.] It is a potent inhibitor of dihydrofolate reductase and is used in cancer chemotherapy. [Blakley The Biochemistry of Folic Acid and Related Pteridines, North-Holland Publ Co., Amsterdam, NY, pp157-163 1969, Beilstein 26 IV 3833.] It is CARCINOGENIC; HANDLE WITH EXTREME CARE.
3.3 Shipping
UN1544 Alkaloids, solid, n.o.s. or Alkaloid salts,solid, n.o.s. poisonous, Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required. UN2811Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
3.4 Usage
Used as a antineoplastic and antirheumatic. A folic Acid antagonist
3.5 Waste Disposal
It is inappropriate and possibly dangerous to the environment to dispose of expired orwaste drugs and pharmaceuticals by flushing them downthe toilet or discarding them to the trash. Household quantities of expired or waste pharmaceuticals may be mixedwith wet cat litter or coffee grounds, double-bagged inplastic, discard in trash. Larger quantities shall carefullytake into consideration applicable DEA, EPA, and FDAregulations. If possible return the pharmaceutical to themanufacturer for proper disposal being careful to properlylabel and securely package the material. Alternatively, thewaste pharmaceutical shall be labeled, securely packaged and transported by a state licensed medical waste contractorto dispose by burial in a licensed hazardous or toxic wastelandfill or incinerator.
4.Safety and Handling
4.1 Symbol
GHS06, GHS08
4.1 Hazard Codes
T
4.1 Signal Word
Danger
4.1 Risk Statements
R61;R25;R36/38
4.1 Safety Statements
S53;S26;S36/37;S45
4.1 Exposure Standards and Regulations
The Approved Drug Products with Therapeutic Equivalence Evaluations List identifies currently marketed prescription drug products, incl methotrexate sodium, approved on the basis of safety and effectiveness by FDA under sections 505 of the Federal Food, Drug, and Cosmetic Act. /Methotrexate Sodium/
4.2 Packing Group
III
4.2 Octanol/Water Partition Coefficient
log Kow = -1.85
4.3 Fire Hazard
Flash point data for Methotrexate are not available; however, Methotrexate is probably combustible.
4.4 Hazard Class
6.1(b)
4.4 Hazard Declaration
H301-H315-H319-H340-H360
4.4 DisposalMethods
SRP: The most favorable course of action is to use an alternative chemical product with less inherent propensity for occupational exposure or environmental contamination. Recycle any unused portion of the material for its approved use or return it to the manufacturer or supplier. Ultimate disposal of the chemical must consider: the material's impact on air quality; potential migration in soil or water; effects on animal, aquatic, and plant life; and conformance with environmental and public health regulations.
Product Application of Methotrexate CAS#59-05-2
1. The treatment of leukemia used to be administered daily, with adults taking 2.5 to 10 mg/d orally, with a total dose of 50 to 150 mg. Children 1.25~5mg/d, recent trends tend to use a large number of intermittent dosing therapy, dosing therapy, that is, oral or intramuscular injection twice a week, 0.25~0.75mg/kg each time, adults generally 20~25mg each time, intrathecally Injection: 10 to 15 mg/time; children use 6 to 12 mg/time according to age, once a day for treatment, for 3 days; once every 4 to 8 weeks for prevention.
2. For chorioepithelial carcinoma, adults generally take 10 to 30 mg, orally or intramuscularly, once a day for 5 consecutive days. The course of treatment can be repeated later depending on the patient's response.
3. For solid cancer, it is best to use continuous intra-arterial infusion and at the same time give intermittent intramuscular injection of leucovorin (CF). The usual dosage is 25-50mg/d of this product, 6-9mg of CF, 1 intramuscular injection every 4-6 hours. Second-rate.
4. For osteosarcoma, use high doses and cooperate with CF detoxification. Generally, the dosage of this product is 3~20g/m2, dissolved in 500~1000ml of 5% glucose injection, and infused intravenously for 4 hours. Start applying CF 2 to 6 hours after the infusion, with a dose of 6 to 12 mg intramuscularly (or orally), once every 6 hours for a total of 3 days. In order to ensure that the drug can be quickly excreted from the body, electrolytes, water and sodium bicarbonate should be supplemented in the first 1 day and every 1 to 2 days of infusion, so that the urine output exceeds 3000ml per day, and ensure that it is alkaline. Liver function, kidney function, blood images and plasma methotrexate concentration should be tested daily.
5. It is rarely used to treat psoriasis due to severe side effects. To treat psoriasis, take 1.25mg orally each time, 2 to 3 times a day. A course of treatment lasts for 6 to 9 days.
Factory and Equipment Show


Fast delivery time
Inventory 2-3 working days New production 7-10 working days

FAQ
Q1: Can I get some samples?
A: Yes, we can supply you with the free sample.
Q2: How to start orders or make payments?
A: Proforma invoice will be sent firstly after confirmation of order, enclosed our bank information. Payment by T/T, Western Union and so on…
Q3: How to confirm the Product Quality before placing orders?
A: You can get free samples for some products, you only need to pay the shipping cost or arrange a courier to us and take the samples. You can send us your product specifications and requests, we will manufacture the products according to your requests. You also could send samples for us to testing.
Q4: What’s your MOQ?
A: Different products with different MOQ, but we accept your any requests of the quantity.
Q5: How about delivery lead time?
A: Inventory 2-3 working days, new production 7-10 working days after payment confirmed. (Chinese holiday not included)
Q6: Is there a discount?
A: Yes, we can give you the most competitive price than any other official quote.
Q7: How to contact us?
A: You can chat with us online or by WhatsApp +86-188-6575-9396.
You can also choose your interested products then send inquiry to us by e-mail inquiry@sincerechemical.com, or call us directly.
Then you will get reply from us within 24 hours.






![5-{4-[2-(5-Ethyl-2-pyridyl)ethoxy]benzyl}-2-imino-4-thiazolidinone CAS#105355-26-8](https://sdluxicdn.huazhi.cloud/cdn/ff/VfHfUxYWPG7mNbVnwauG7oHjykFXiFceYP1paSqb7zU/1718165820/public/styles/chanp/public/2024-06/%E7%99%BD%E8%89%B2%E7%B2%89%E6%9C%AB%20%282%29_4.jpg?itok=tBW6CWQB)
